Determine Whether or Not Each Hydrocarbon Is Saturated.
This is known as the Baeyer Test for unsaturation. A pink to purple colour depending on concentration.
Illustrate Saturated And Unsaturated Hydrocarbons Chemistry
Each tube and whether or not hydrogen bromide was evolved and record the results.

. You assess the textdegree of unsaturation An hydrocarbon of formula C_nH_2n2 is said to be textSATURATEDie. Identify whether each of these hydrocarbons is saturated or unsaturated. We can see that each carbon has all four covalent bonds so the answer is that the hydrocarbon is saturated.
Place a U for unsaturated next to each hydrocarbon letter that is unsaturated. Meaning it is saturated when we say saturated were saying it is saturated with hydrogen bonds meaning itll only form single bonds going all the way across. The saturated hydrocarbon alkanes and cycloalkanes cycloalkane pentane and heptane.
As you can see there are several double bonds in each hydrocarbon chain so its definitely unsaturated. Whether there are double or other multiple bonds in the molecule or not. True or false A saturated hydrocarbon has only single bonds.
Place an S for saturated next to each hydrocarbon letter that is saturated. Place an S for saturated next to each hydrocarbon letter that is saturated. Unsaturated hydrocarbons ie alkenes and alkynes react by addition of reagents to the double or triple bonds.
Try it out for methane ethane propane etc. Add 3 drops of 1 aqueous KMnO4 to each test tube swirl gently and let stand. A Determine the number of hydrogen atoms in a.
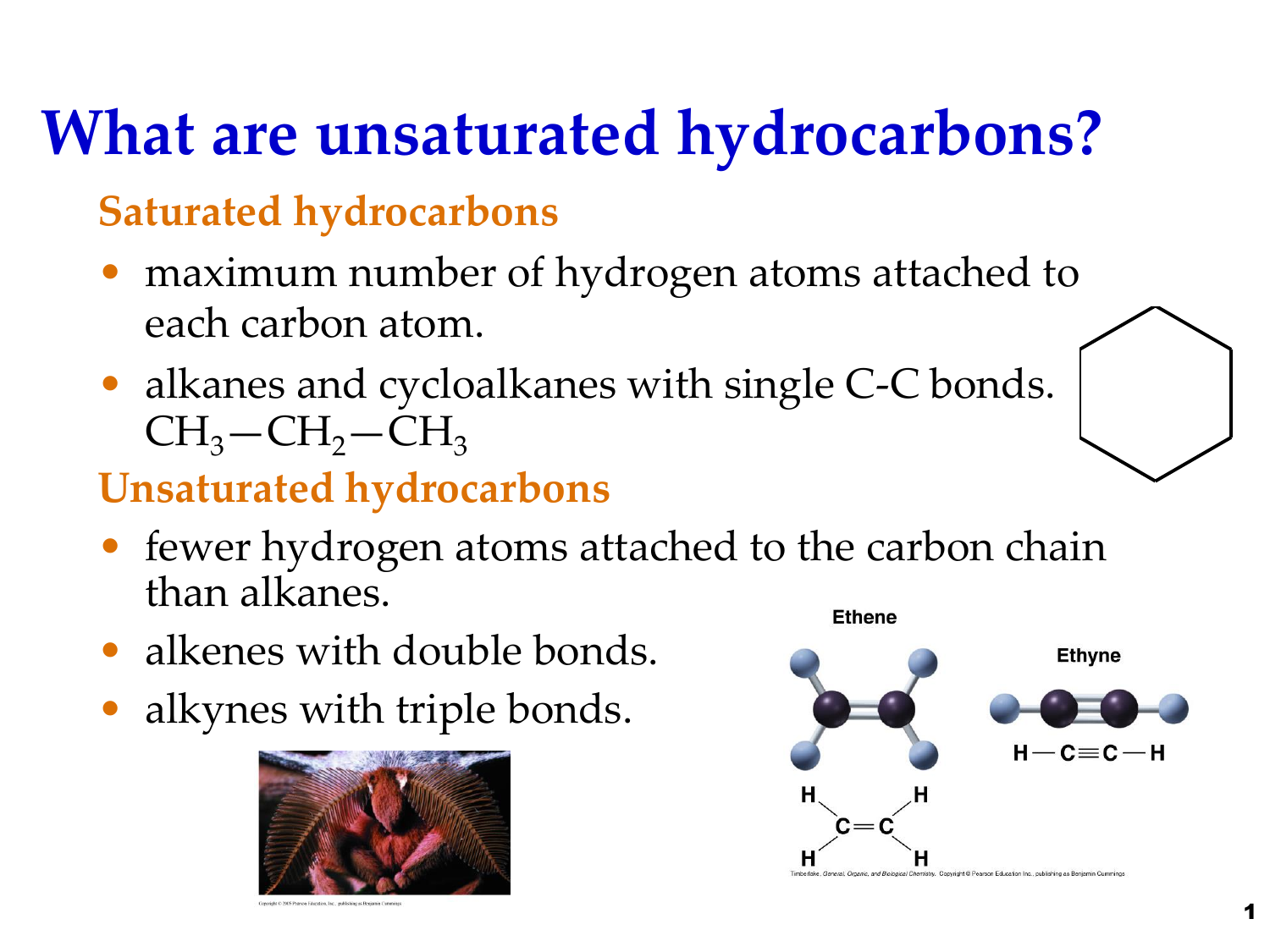
Usually this refers to bonds between pairs of carbon atoms. Saturated hydrocarons contain single bond between carbon atoms. Correct option is B Always suffix -ene contains double bond between carbon atoms and in case of -yne it contains triple bond between carbon atoms where as in case of -ane it contains single bond between carbon atoms.
Bromine Water Test. We need to look in this hydrocarbon tail and determine if there are any double bonds. A Saturated hydrocarbon is a hydrocarbon in which all the carbon-carbon bonds are single bonds.
A saturated hydrocarbon is a hydrocarbon in which all carboncarbon bonds are single bonds. Both types of bonds can be hydrogenated with. As the name suggests saturated hydrocarbons are hydrocarbons in which all the carbon atoms are bonded to four other atoms and are saturated implying that no carbon.
Calculating the mass or concentration of each component in a substance D Removing. A saturated hydrocarbon contains only single covalent bonds b. Use the general formulas for alkanes alkenes and alkynes to answer the following questions and indicate whether the hydrocarbon is saturated or unsaturated.
Place 1 ml of each of the hydrocarbons heptane pentene toluene and the unknown into separate clean and dry small test tubes. In essence each carbon has four single covalent bonds. Answer 1 of 2.
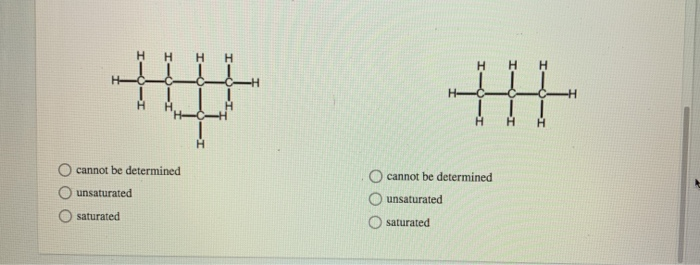
The general formula for a fifteen carbon alkane would be C15H30 C. To determine if it is saturated monounsaturated or poly unsaturated. Answer to Solved Determine whether or not each hydrocarbon is.
A saturated hydrocarbon is one where all the carbon atoms cant form any more bonds. Shake the mixture to determine whether the hydrocarbon is soluble a colorless second layer may. B Aqueous Potassium Permanganate Baeyers Test In a small test tube add 1 ml 10 drops.
Answer each of the following statements as true or false. Determine whether or not this hydrocarbon is saturated or unsaturated A Saturated B Unsaturated. Of hydrocarbon to a mixture of 3 ml.
Hence here benzene is not a saturated hydrocarbon. The chemical reactivity of hydrocarbons is determined by the type of bond in the compound. Each TWO hydrogens LESS than the saturated formula corresponds to.
Potassium permanganate solution KMnO 4 aq. Identify whether each of these hydrocarbons is saturated or unsaturated. Saturated hydrocarbons are the simplest type of organic compound.
An unsaturated hydrocarbon is a hydrocarbon in which one or more carboncarbon multiple bonds double bonds triple bonds or both are present. Determine whether or not each hydrocarbon is saturated Saturated compounds have as many hydrogens around each carbon as possible which is achieved by only having single bonds present. We have just a hydrocarbon chain you know even though hydrocarbon chains by themselves.
The addition products become saturated with fragments of the reagent becoming attached the carbons of the multiple bond. Ii 14 carbon alkene. 2 central carbons with C H 3 at each end and also single bonded to the first carbon.
Observe the color of each tube and whether or not hydrogen bromide was evolved and record the results. In order for an organic compound to be classified as. There are no double bonds here.
Chemistry questions and answers. Of dilute sodium carbonate solution 10 Na 2 CO 3 solution and. Thus the two structures that only have single bonds are saturated.
Answer 1 of 12. Experts are tested by Chegg as specialists in their subject area. The preferred test for unsaturation uses bromine water Br 2 aq 4.
100 62 ratings Transcribed image text. It contains the maximum number of C-H bonds. Unsaturated hydrocarbon have the presence of a double or triple bond while saturated hydrocarbons have single bonds.
Determine whether or not this hydrocarbon is saturated or unsaturated A Saturated. Canes are insoluble in water. B Determine the number of carbon atoms in a.
Alkanes - Are saturated hydrocarbons that therefore contain only hydrogen and carbon atoms bonded to each other and typically follow the chemical formula C n H 2n2. Of dilute potassium permanganate solution 05 KMnO 4 solution and 3 ml. Bromine water Br 2 aq.
Theyre not a bio molecule so theyre not classified as a lipid. Two common reagents used to detect whether a hydrocarbon is saturated or unsaturated are. Place a U for unsaturated next to each hydrocarbon letter that is unsaturated.
A common example is paraffin. A yellow to brown colour depending on concentration. Determine whether or not each hydrocarbon is saturated.
He will be here. KMnO4 reacts with the hydrocarbon a brown colour will be observed MnO 2. How are unsaturated hydrocarbon different from saturated hydrocarbon.
Determine whether or not each hydrocarbon is saturated. A hydrocarbon is an organic compound whose only constituents are carbon and hydrogen. But there was no Sal.
Alkenes - These unsaturated hydrocarbons are molecules that contain at least one carbon-to-carbon double bond. Lets look at more. We review their content and use your feedback to keep the quality high.
Vinylic double or acetylenic triple carbon-carbon bonds.
Which Hydrocarbons Are Saturated Which Ones Are Unsaturated Quora
Difference Between Saturated And Unsaturated Hydrocarbons Definition Structure Types Properties
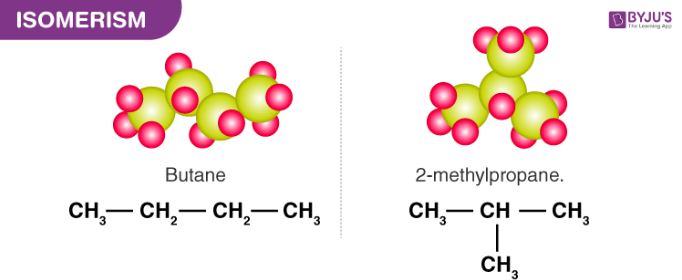
Saturated Hydrocarbon Detailed Explanation With Examples

Saturated Hydrocarbon Ck 12 Foundation
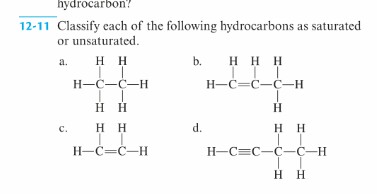
Solved Hydrocarbon 12 11 Classify Each Of The Following Chegg Com

Saturated Hydrocarbon Definition Examples Video Lesson Transcript Study Com

Which Hydrocarbons Are Saturated Which Ones Are Unsaturated Quora
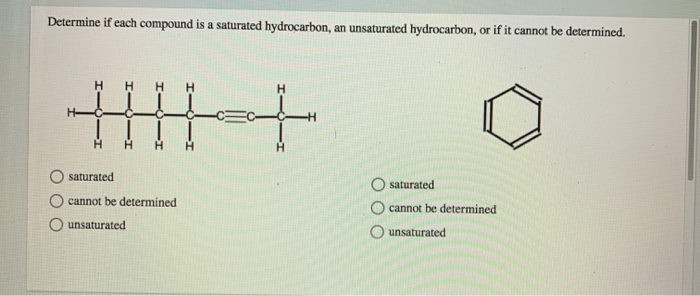
Solved Determine If Each Compound Is A Saturated Chegg Com
What Is A Saturated Hydrocarbon Quora

An Introduction To Organic Chemistry The Saturated Hydrocarbons Ppt Video Online Download

What Are The Different Types Of Hydrocarbons Quora
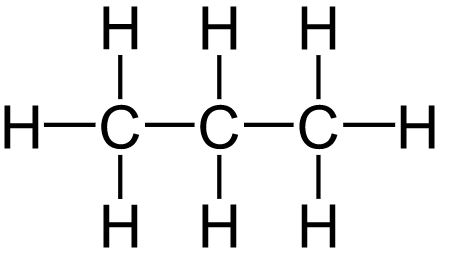
Saturated Hydrocarbon Detailed Explanation With Examples
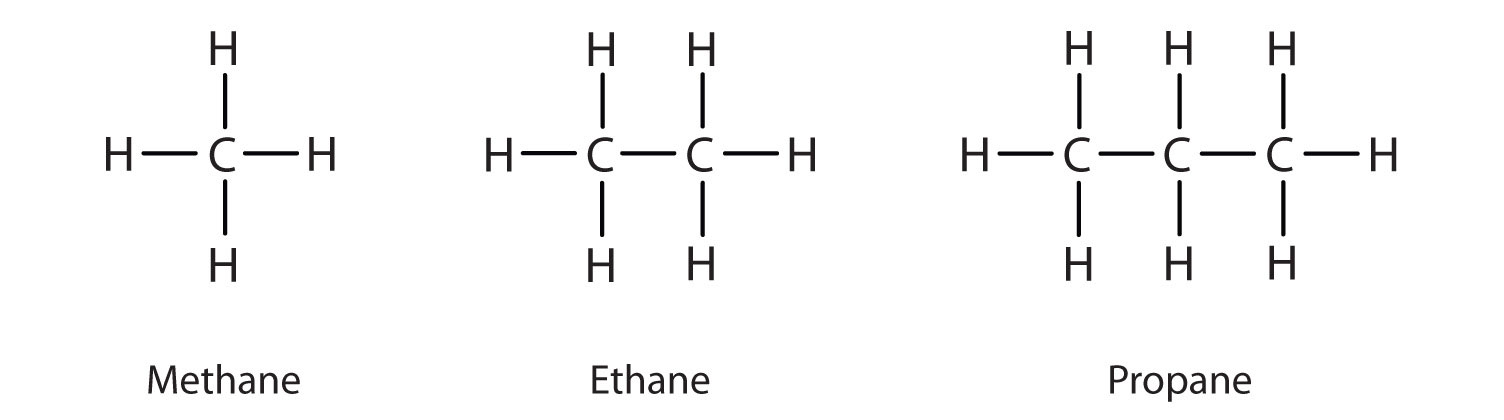
10 4 Alkanes Saturated Hydrocarbons Chemistry Libretexts
Which Hydrocarbons Are Saturated Which Ones Are Unsaturated Quora
Comments
Post a Comment